What cabling installers need to know about building fires
Why is firestopping important? The more you know about how a building fire develops, the more you`ll understand why.
Joseph R. O`Brien, Nelson Firestop Products
Firestops play an important role in passive fire containment, which, in turn, is one of the key strategies in reducing fire losses. Knowledge of how a fire develops is necessary for a full understanding of containment and firestopping. Important factors to consider are how solids burn, how fire develops within a compartment, how fire spreads, how fast it spreads, and how fire kills.
Fire is defined as the rapid oxidation of a fuel. The reaction is exothermic, which means that heat is released. For the reaction to exist, there must be sources of fuel, oxygen, and heat.
Fuel is supplied by the materials at the fire scene. A practical method of controlling the size of a fire is to limit the exposure of fuel by compartmentalizing buildings.
Oxygen is normally supplied by atmospheric air. Because oxygen is consumed rapidly, the supply must be continuously replenished. One effective technique for fire suppression is to interfere with the oxygen supply--for example, by use of carbon dioxide or Halon extinguishing systems.
Heat is supplied initially by an ignition source, such as a match, a cigarette, or a defective electrical circuit. Once the fire is established, it may be able to supply its own heat at a high enough rate to sustain it or even promote growth. Water extinguishing systems chill the fire, eliminating the heat necessary to sustain the reaction.
Fueling the fire
In accidental fires, most of the fuels are solid, organic materials. These may be natural or man-made, but they all share a common chemistry. Carbon, hydrogen, and oxygen are the building blocks for virtually all combustibles.
Solids do not burn directly. Heat radiating onto the surface of the solid causes thermal decomposition, releasing vapors rich in combustible hydrocarbons and leaving behind a residue of char or ash that may continue to decompose as the fire progresses. This process is called pyrolysis, and the fuel vapor is known as pyrolyzate. Combustion actually takes place in the vapor phase, during which atmospheric air can mix freely with the pyrolyzate.
Thermal gradients caused by the fire produce an updraft, allowing cool air to flow to the base of the flame. This replenishes the oxygen supply. A high degree of turbulence soon develops, helping entrain more air and mixing it more thoroughly with the fuel. Extremely powerful winds can be produced by the fire as it draws in the needed air supply.
As the fire develops, hot, smoky gases rise. Inside a compartment, the gases form a layer just below the ceiling. This layer has a fairly distinct, visible boundary with the cooler atmosphere below. Both the depth and temperature of this hot layer grow rapidly. Heat radiates directly from the flame and also from the hot layer, raising the temperature of other combustibles in the vicinity. These combustibles then begin to pyrolyze, contributing additional fuel.
Various materials generate heat at different rates. For a given material, the heat-release rate determines the amount of heat available to pyrolyze additional fuel per unit of time. Heat release rate, then, is one possible measure of the degree of hazard associated with a material or product.
Air flow needed
Atmospheric air is the main source of oxygen for most fires. However, even a small fire would quickly exhaust the oxygen in a typical room. For example, a large upholstered chair burning rapidly could consume all the oxygen in an average-sized house within approximately 30 seconds.
Clearly, no fire could ever reach dangerous size in a completely airtight compartment. Unfortunately, no life could exist in that compartment, either. Practical, real-world compartments are leaky, and pressure and thermal gradients quickly establish the air flow necessary to support some level of combustion. The effective size of the openings available for air flow determines how much oxygen can reach the fire.
The restriction of air supply limits the ultimate size of the fire. Consider a room fire where the only opening is an open window. Initially, the entire opening is available to admit more air. As the hot layer against the ceiling deepens, it reaches the top of the window, and hot, smoky gas begins to flow out. As the hot layer continues to grow, more and more of the area of the window is used for the outflow of smoke, and an increasingly less area is available for the admission of fresh air. Oxygen depletion then limits the further growth of the fire.
Fire containment
Fires can grow with terrifying speed, and fire fighting becomes exponentially more difficult as the size of the fire increases. Because it takes time for the fire department to reach the scene and begin operations, fire containment is an important initial fire-fighting strategy. Containment limits the size of the fire and its rate of growth by isolating potential fuel sources and regulating the air supply.
Containment is provided by properly designed and installed fire-rated walls, floors, doors, windows, dampers and firestops. These partitions are the most important line of defense against fire, because they do not rely on outside sources of energy to perform their functions. Automatic sprinkler systems have an outstanding record as fire suppression systems--when they are in working order. But they are dependent upon the available water supply and in many cases, the startup of a fire pump. Some spectacular fire losses have resulted from the failure of these services--notably the total loss of Chicago`s McCormick Place Exhibition Center due to the failure of the water supply.
History of a fire
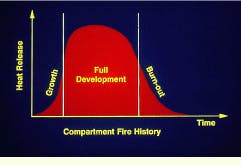
To effectively plan for containment, it is useful to understand the life cycle of a fire. From the moment of ignition, a typical compartment fire goes through a period of rapid growth, a stage of fairly uniform development limited by either fuel or oxygen supply, and a final stage of burn-out or flashover. Without proper containment, burnout may be preceded by spread to adjacent areas.
The early development of a fire may take only 30 sec. Once ignition occurs, heat from the flame radiates back to the fuel surface, causing additional pyrolysis. New fuel vapors rise from the surface, entraining atmospheric air and igniting. If the radiant energy from the flame can pyrolyze at least as much new fuel as the flame consumes, the fire will be self-sustaining.
In the early phase, the flame is relatively small and air can mix with fuel, promoting complete combustion. At this point, the combustion by-products may be principally carbon dioxide and water.
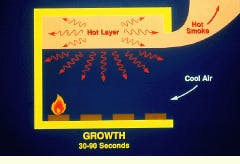
The growth phase of a compartment fire may occur 30 to 90 sec following ignition. As the fire grows and flames cover a larger surface area, it becomes difficult for air to mix uniformly in the interior portion of the flame. Due to incomplete combustion, the smoke contains rising levels of carbon monoxide and possibly other toxic gases, as well as aerosols and soot.
Hot gases rising toward the ceiling form a hot layer that heats the ceiling and upper walls. As these surfaces become hotter, they begin to reradiate energy, which adds to the radiation from the hot layer itself. This energy, in addition to direct radiation and convection from the flame, raises the temperature of the room contents, which begin to pyrolyze, adding to the fuel supply.
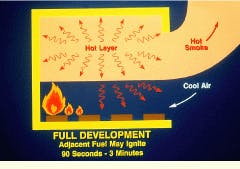
The full development of the fire, during which adjacent fuel may ignite, occurs between 90 sec and 3 minutes following ignition. As long as plenty of air is available, the growth of the fire is primarily limited by the amount of fuel being released. As the fire spreads, the oxygen demand rises, and oxygen depletion may limit the further growth of the fire.
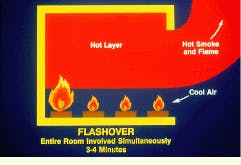
During flashover, the final phase of the fire, the entire room is involved simultaneously. This phase usually occurs 3 to 4 min following ignition. As the temperature of the hot layer rises, increasingly more radiant energy falls on the other combustible materials in the room. It is possible for the surface temperatures of these materials to reach the self-ignition temperature more or less simultaneously. Under these conditions, the entire room contents may suddenly burst into flame. Oxygen depletion prevents the complete combustion of all pyrolysis products, which pour out of the room openings where they immediately burst into flame on contact with air. The huge fireball rolling out of the combustion chamber provides the heat and ignition source for fuels in adjacent chambers.
This extremely dramatic event is a common experience, undoubtedly responsible for the rapid spread of many fires. The National Bureau of Standards, among other organizations, has conducted many room fire tests, using real furnishings and room contents in realistic fire scenarios. The time from ignition to flashover in a typical basement recreation room fire was roughly 3 min in these tests.
As the fire approaches flashover conditions, the temperature of the hot gases emerging from the room is on the order of 1300oF. Carbon monoxide content might be 5%. Hot gases are produced at several cubic meters per second, enough to fill an entire floor with smoke within a few minutes.
Fire intensity
One measure of the intensity of a fire is the amount of heat released, which can be expressed in watts of energy. For reference, a typical incandescent lamp burns about 100 watts, while a toaster might burn at 1600W. By contrast, a large, upholstered easy chair can release energy at the rate of 1 to 3 million watts, depending on the materials used to make it.
The development of the hot layer in a fire is a function of the heat release rate. In a 1-megawatt fire, the hot layer in a 25 x 25 x 8-foot room extends downward 6 feet from the ceiling within 2 min. In a 3-MW fire, the hot layer reaches a depth of 6 feet in less than 1 min.
In many fires, the source of ignition may be a dropped cigarette or an electrical overload. Based on the specific geometry and on the combustible materials present, flaming ignition may not occur. Smoldering may continue for an extended period, even if flaming does eventually develop. For flaming ignition from a smoldering source, the pyrolyzate must accumulate without much air dilution until it can reach ignition temperature. A cigarette that rolls into a crease in upholstery or bedding is more likely to cause a flaming fire than one that falls on a flat surface.
Smoldering fires may generate larger volumes of toxic smoke and gases than equivalent flaming fires. Some highly toxic gases, such as hydrogen cyanide, may be more stable at the lower temperatures of smoldering fires and may, therefore, have more lethal potential than in the case of flaming fires.
Fire deaths
Fire deaths may occur as a direct result of burns, or as a result of smoke inhalation. By far the greatest number--probably more than 80%--are due to smoke inhalation.
Burn victims are usually found near the origin of the fire. Because the fire can grow so rapidly, the environment may become untenable for life within a minute or so after flaming ignition. Skin burns at approximately 180oF. Radiation from the hot layer can produce such temperatures very quickly.
Most fire victims do not suffer burns. Autopsy data is rarely available, so the cause of death is usually reported as "smoke inhalation," a catchall for many possible causes. Smoke is an inclusive term for combustion by-products, which may encompass toxic gases, irritants, aerosols, soot, and other particulate matter. Smoke may contribute to death directly through toxic reactions or oxygen reduction, or indirectly by obscuring vision to prevent or delay escape. Disorientation due to alcohol intoxication makes it even less likely that the victim can escape.
All organic materials, whether natural or synthetic, generate toxic gases while burning. Despite the increasing use of synthetic materials, fire death rates are declining. No conclusive evidence has been developed to show that natural materials are less likely to produce toxic fumes.
Carbon monoxide is the only toxic gas involved in common fires, in which the link with fire deaths is indisputably established. Carbon monoxide is present in the smoke from all organic materials. Its lethal potential is well-documented. The gas attaches itself to blood hemoglobin in a way that destroys the hemoglobin`s ability to distribute oxygen to body tissues. A few breaths of air containing 5% carbon monoxide is invariably fatal. At 0.5%, incapacitation may occur within 15 min and death after about 45 min of exposure. Victims with pre-existing heart disease can succumb at even lower levels of exposure.
Lethal levels of carbon monoxide have been found in at least 50% of the fire fatalities studied. In another 30%, combinations of carbon monoxide with either heart disease or alcohol intoxication were blamed. Carbon monoxide, then, has played a major role in approximately 80% of the fire deaths studied.
Other lethal or potentially lethal gases which may be present in smoke include hydrogen cyanide, hydrogen chloride, nitrogen dioxide, sulfur dioxide, ammonia, isocyanates, and acrolein. Because of the nearly universal presence of carbon monoxide, autopsy reports are rarely able to reach any clear conclusion about the effects of these other harmful gases.
Credits
Data for this article has been compiled from information on the National Bureau of Standards (NBS--Washington, DC) Recreation Room Fire Test (1971), one of the many tests the NBS has performed on combustible materials.
The basic information here was outlined by John Birmingham, chief engineer of firestop products at Nelson Firestop Products. After a long and courageous battle with cancer, he passed away on August 25, 1996, while reviewing the final draft of this article. John was a pioneer in firestopping, and we have lost a true friend and colleague who was kind and helpful.
Maximum heat release in a fire occurs during the full-development stage.
Early development of a fire may take as little as 30 sec. The flame may be relatively small at this point, leading to complete combustion of the fuel.
During the growth phase of a fire, smoke and toxic gases are often released because of incomplete combustion.
Full development of a compartment fire may occur 90 sec to 3 min following ignition. As long as there is plenty of air available, the growth of the fire is primarily limited by the amount of fuel being released.
During flashover, the final phase of a compartment fire, the entire room is involved simultaneously.
Joint testing
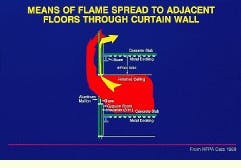
The testing of flexible joint seals, developed to accommodate movement caused by seismic or other loading, is covered by UL-2079 (Underwriters Laboratories, Northbrook, IL). The American Society for Testing and Materials (astm--West Conshohocken, PA) has yet to publish a standard for joint testing; however, such a standard is in the final stages of development. Perimeter-seal curtain-wall joint testing is a major firestopping issue that does not fall directly under the joint-testing standard, due to the complex dynamics and reduced fire ratings associated with curtain-wall assemblies. An astm standard for curtain-wall perimeter-seal joint testing is in the early stages of development.
In a building with curtain walls, the fire can leap from floor to floor as windows break because of the intense heat.
Joseph R. O`Brien is an expert on firestopping with Nelson Firestop Products (Tulsa, OK). He has been involved with bicsi since 1974, serving on its Engineering and Standards Committee. O`Brien is active on the eia/tia Standards Committee on Firestopping and in the National Fire Protection Association.